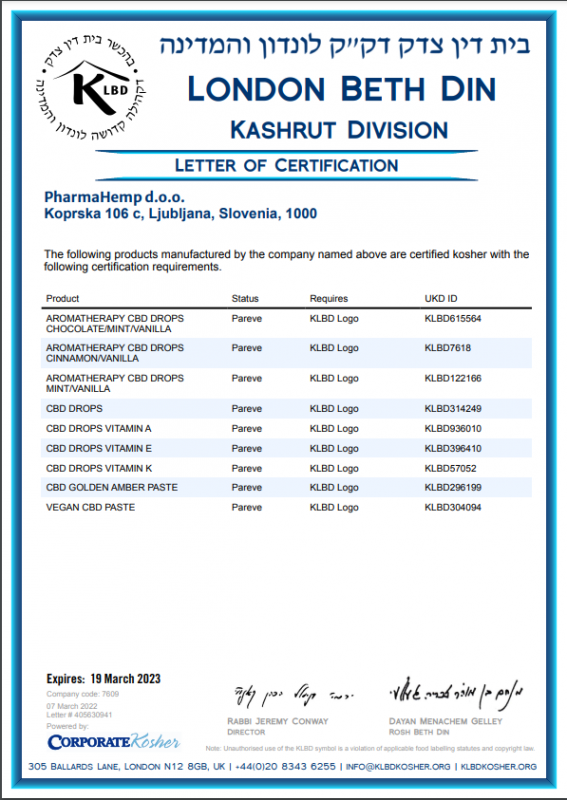
integrated quality ASSURANCE
Our promise is to make everyday life better by developing a sustainable culture in the industrial hemp industry and the highest quality standards. The efficiency, safety and quality of our products and services are our commitment to our customers. We ensure quality business operations and the continuous improvement of all processes. We follow GxP good business practice guidelines (GACP, GDP, GLP, GMP) and certified management systems, evidenced by independent certification agencies.


 Slovenščina
Slovenščina Deutsch
Deutsch Español
Español